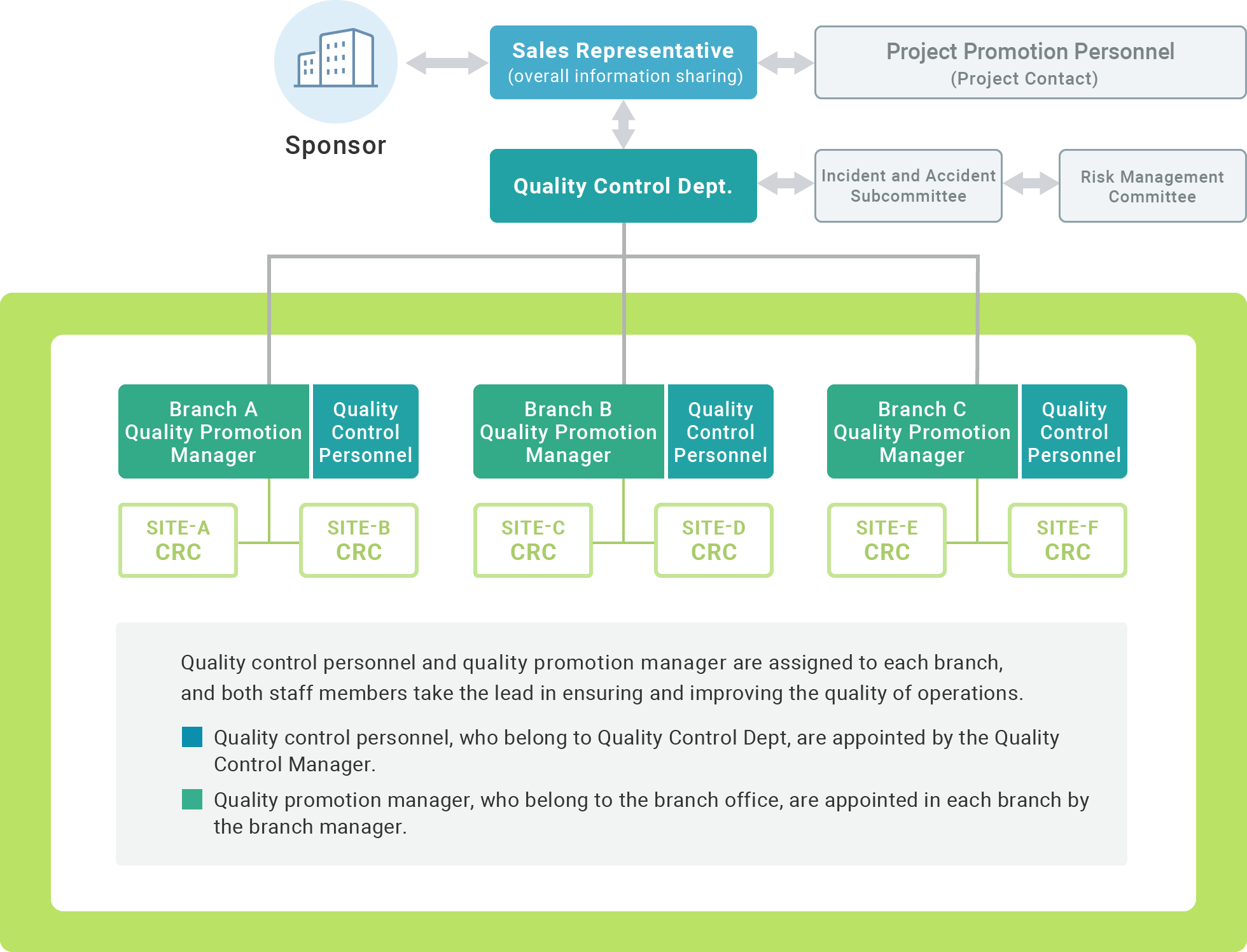
We have established a Quality Control Department as a specialized division responsible for our quality control. The Quality Control Department maintains and operates the Standard Operating Procedures for clinical trial site support operations, as well as manuals and operational tools for each type of work. In addition, it is responsible for specialized education (education related to the commissioning and implementation of site support services) and work to improve and homogenize the quality of site support services throughout the company through mutual collaboration with each branch and relevant departments.
We support and promote risk-based process management at medical institutions to ensure the quality of clinical trial data.
Our CRCs, Site Management Associates (clinical trial secretariat staff) and business development staff for sites support the establishment of a clinical trial implementation system and processes, in consultation with the investigator and site staff. In addition, quality control staff and a quality promotion manager visit the site and inspect from a third party's perspective to ensure that clinical trial operations are being conducted in accordance with the process.
The Quality Control Department centrally manages company-wide incident information through a reporting system. When an accident occurs, the department responds promptly and appropriately in cooperation with related parties and relevant departments. After an accident occurs, the Quality Control Department investigates and analyzes the incident/accident and the factors behind the case, and a specialized committee (Incident/Accident Subcommittee) investigates the cause of the case, examines measures to prevent recurrence, and promptly implements these measures in order to prevent recurrence.
We are committed to compliance and ensuring the reliability of clinical trial data.