
Electronic management cloud system for clinical trial documents
to improve operational efficiency and quality
Do you have any of these problems?
-
Printing and filing require a huge amount of work.
-
It is difficult to secure document storage space.
-
Want to reduce postal and visit costs for document exchange.
-
System implementation is a burden with a large initial cost.
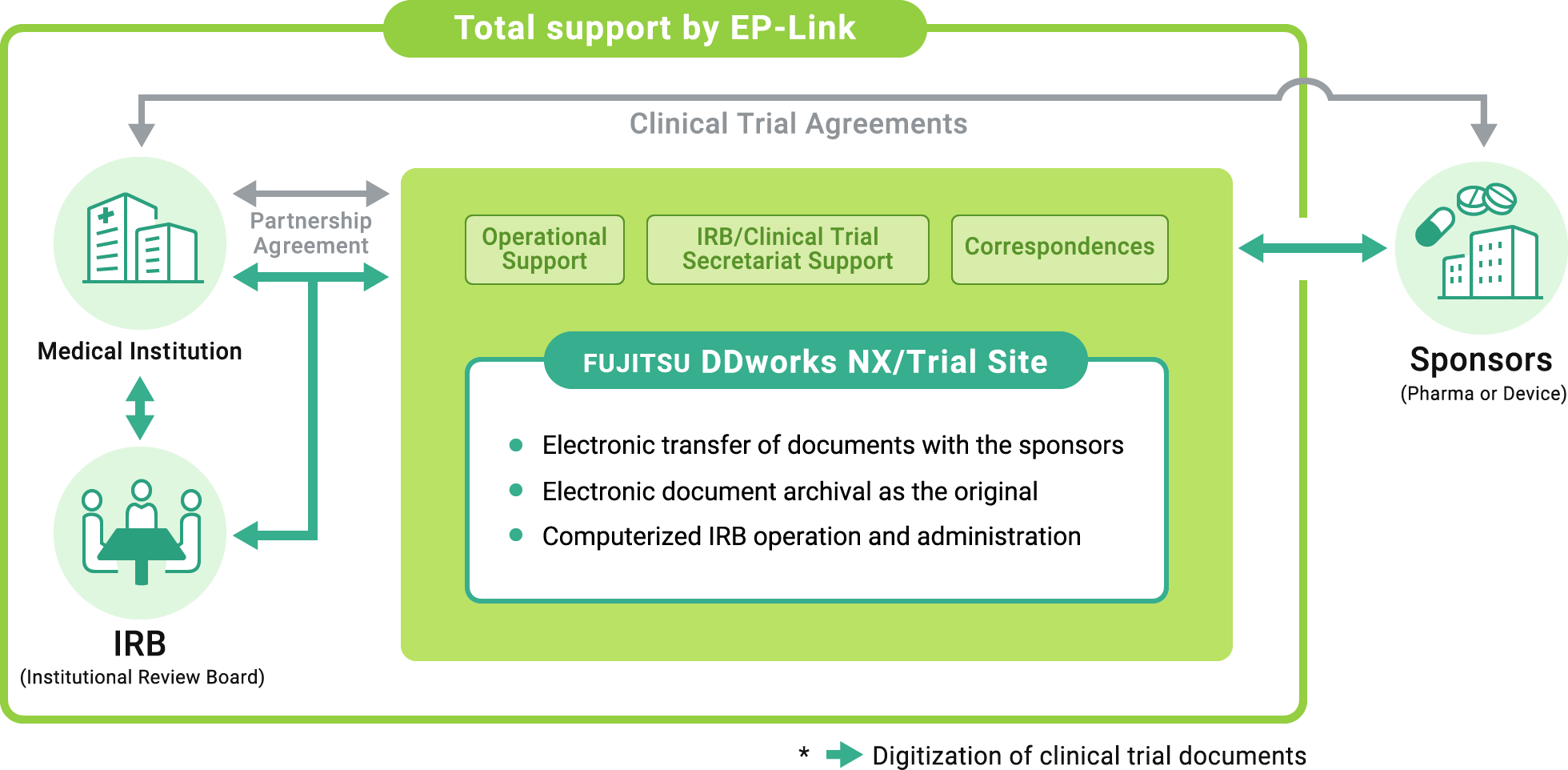
EP-Link's services contribute to improving operational efficiency by converting various electronic data used in clinical trials into original documents and digitizing related business processes, providing many benefits such as cost reduction and improved information security.
Advantages of DDTS
-
Reduce the enormous amount of document management work and costs
When documents are received, they can be labeled and stored in an organized state in the system, greatly reducing the man-hours (costs) associated with filing.
-
Easy and speedy transfer of documents
As documents are exchanged on the cloud, the IRB application document and the IRB determination letter can be automatically created and issued within the system.
-
E-mail (Q&A) within DDTS
Queries and responses can be recorded within the system for each site. You can also easily search for past interactions.
-
Streamline document viewing and search to avoid the risk of document loss
IRB (Institutional Review Board) related documents are stored in the cloud, so they can be viewed remotely and directly. Since documents are not moved, there is no risk of loss, and there is no need to arrange and reserve a reading room.
-
Significantly reduces document storage space
Since all documents such as protocol, Investigator's Brochure, and safety information are stored electronically, the storage space of clinical trial documents can be greatly reduced.
-
Digitization of IRB review materials
Since IRB review materials can be checked on the cloud, it is possible to reduce the time and complexity of shipping and destroying materials to be distributed to IRB committee members and the risk of information leakage.
Key Features of DDTS
- Document storage management
-
- Manage clinical trial documents on the Internet cloud
- Compatible with both electronic and scanned paper files
- Electronic document exchange and Q&A management
-
- Electronic exchange of documents in both directions between pharmaceutical companies and medical institutions
- Q&A management with pharmaceutical companies, etc. (list and accumulate*)
- In-hospital workflow
-
- Electronic review/approval of documents by relevant personnel within the medical institution (e.g., CRC→ Principal Investigator, Secretariat → Principal Investigator)
- Management functions for deviations (preparation of reports, confirmation by Principal Investigator, preparation of lists, etc.)
- IRB (Institutional Review Board) Management
-
- Batch electronic reception of review requests, automatic completion of Form 4, assistance in the completion of Form 5, output of drafts for unified formats
- Preliminary review of materials by the IRB
- Compliant with regulations on digitization (ER/ES Guidelines, 21 CFR Part 11)
- Extensive CSV (Computer System Validation) experience
- Winner of the overall grand prize at the ASP/SaaS/Cloud Awards 2014
- Providing reliable support
- ISO27001 (ISMS certification)* Awarded
- Appropriate information management is in place to prevent unauthorized access to information systems, virus infection, etc.
- Data center safety and security measures have been implemented.
- Security measures and measures to improve the reliability of cloud systems (equipment configuration, capacity evaluation, backup, operation, etc.) have been implemented.


.png)
@2x.png)
@2x.png)
.png)
@2x.png)
@2x.png)
.png)