
Patient Centricity
Supporting Drug Development Based on Patients' Voices
Patients' Voices
-
"Due to illness and the environment in which I live, it is difficult for me to make regular (frequent) visits to the hospital."
-
"I don't want to burden my family with the transportation."
-
"I would like to reduce hospital visits in terms of infection control."
EP-Link supports DCT promotion.
-Clinical trials that do not rely on visits to the site-
The spread of the concept of Patient Centricity and the use of digital technology will decentralize clinical study-related activities, reduce the frequency of hospital visits, and enable patients who could not previously participate due to disease or circumstance to participate. Clinical studies can be conducted in a patient-centered setting that does not depend on visits to the hospital by conducting the clinical trial activities stipulated in the protocol at the family doctor's office or at the subject's home, etc.
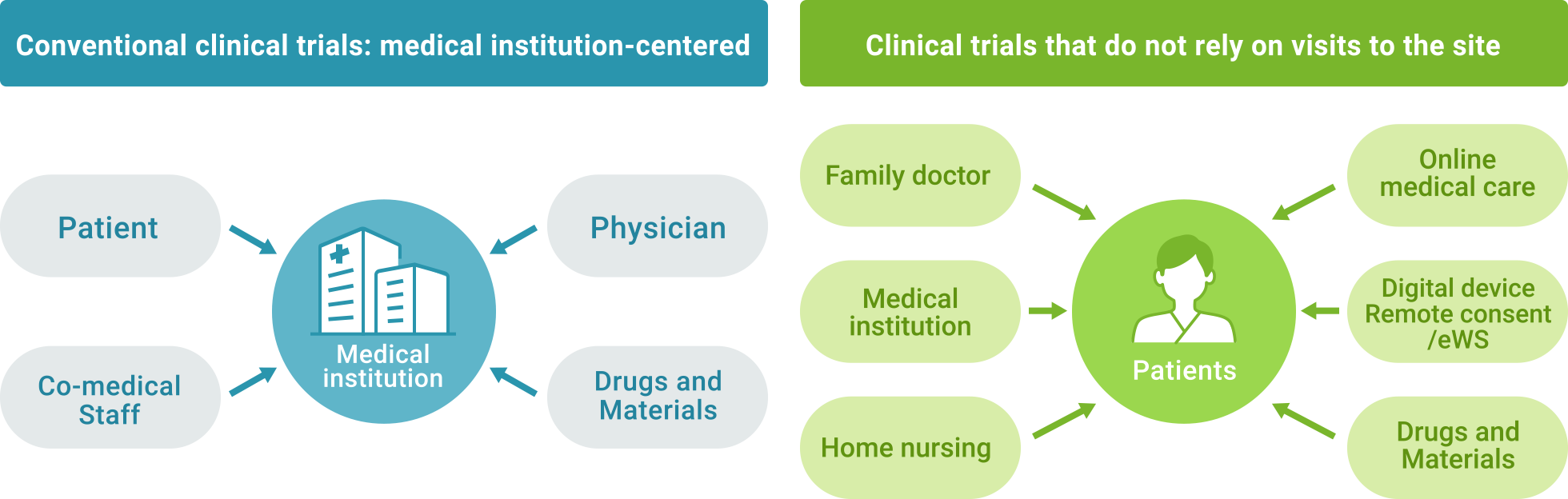
From institution-centered to patient-centered clinical trials
EP-Link's DCT Support
EP-Link offers the most appropriate DCT scheme.
-
Make family doctors' facilities partner medical institutions
By making sites supported by EP-Link across the country partner medical institutions, EP-Link makes it easier for patients to access clinical trials and promote enrollment.
-
Online medical care
Aiming to improve convenience and satisfaction for subjects, EP-Link supports the establishment and operation of an optimal online medical care system for clinical trials, while complying with laws and regulations.
-
Home nursing
Covering a wide range of regions, EP-Link flexibly responds to your needs, working with home nursing staff with expertise in clinical trials, etc., as well as medical institutions and family doctors.
-
Digital devices
Remote consent and eWorksheetEP-Link flexibly supports various systems with its extensive experience in using eConsent and eWorksheet.
-
Drugs and materials
EP-Link supports the smooth implementation of delivery of investigational drugs to subjects' homes, management of test materials, and transportation of samples.
Key points of EP-Link's DCT support
EP-Link's DCT scheme, which boasts a wide range of disease areas and the industry's largest network of medical institutions covering the entire country, brings you:
- Opportunities for patients who have had difficulty participating in clinical trials due to geographical factors to participate in clinical trials.
- Improvement in subject enrollment and operational efficiency.

Supporting patient-centered clinical trials. EP-Link supports clinical trials in the homes of subjects for whom hospital visits are burdensome.
with you to maximize the benefits for all stakeholders.


.png)
@2x.png)
@2x.png)
.png)
@2x.png)
@2x.png)
.png)