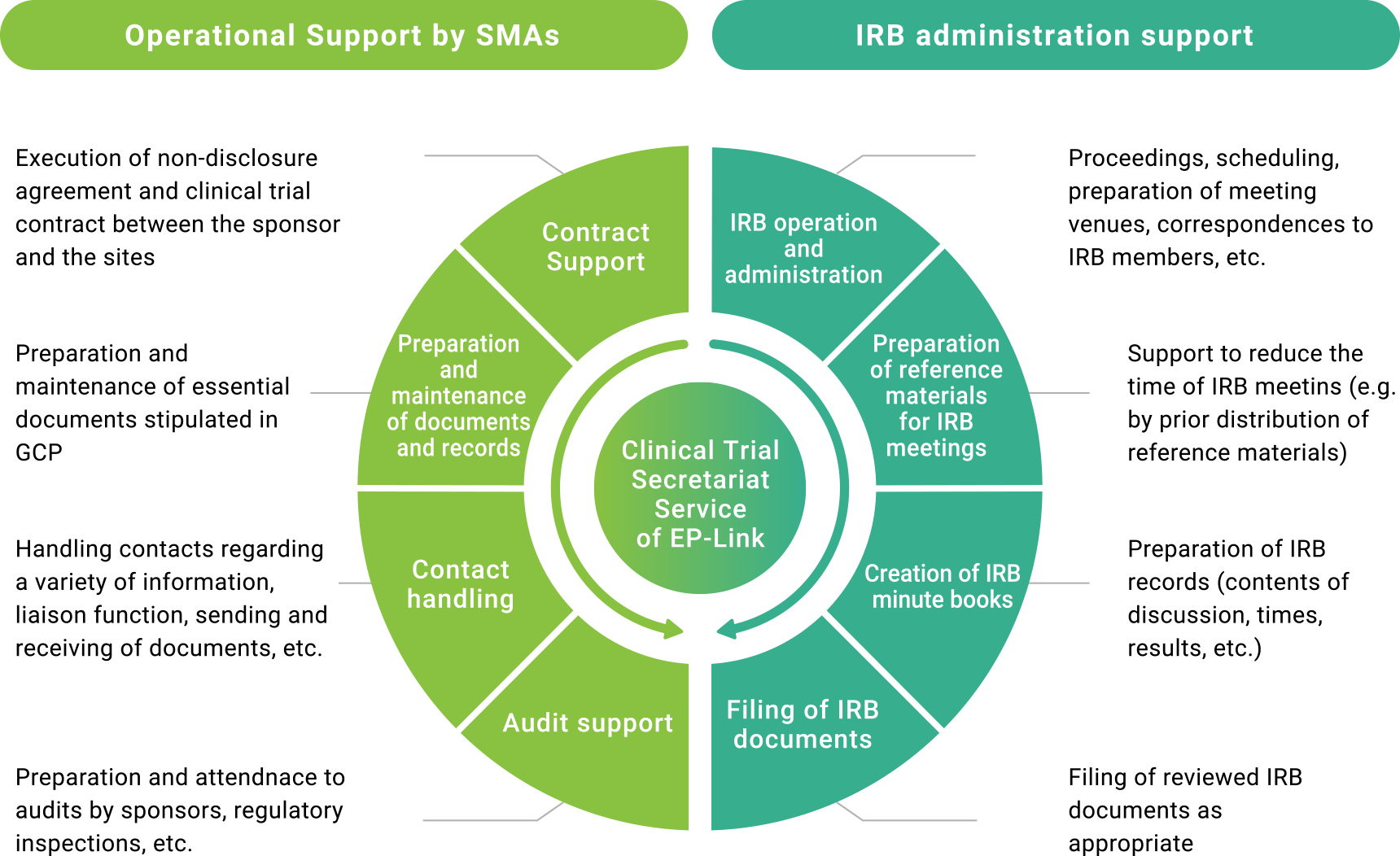
SMA provides total support for the creation of the necessary environment for clinical trials, including support for the establishment of clinical trial secretariat and IRBs, preparation of documents using formats in accordance with the GCP regulation, and education to ensure all clinical trial personnel understand the purpose and content of GCP. Depending on a variety of needs, EP-Link supports you totally or for a single task.
EP-Link not only creates and stores documents required for clinical trials but also provides optimal support depending on whether the site has a local IRB or uses a central IRB* for a review. In addition to pharmaceutical and medical device clinical trials, EP-Link also provides administrative support for clinical research and clinical trials for products such as regenerative medicine. Also, according to your request, EP-Link provides you with different options such as digitization or paperless documentations.
These options are popular because it leads to reducing IRB members’ burden, creating more space in buildings of clinical sites, saving resources and protecting environment.
* Central IRB: IRB set up at a certain site that undertakes reviews of studies for multiple clinical sites in batches.
EP-Link enhance the skills of SMAs and standardizes their quality by providing regular training, extraordinary training in response to regulatory changes, and sharing know-how and knowledge across the organization through case study meetings. Seventy percent of EP-Link’s SMAs are certified by the Japan SMO Association. Based on their extensive operational experience, they respond quickly and accurately to sudden changes in the system of medical institutions.
EP-Link pioneered the establishment of Central IRBs in Japan in 2001 and now supports five Central IRBs. EP-Link has also supported Central IRB reviews entrusted by large scale hospitals such as university hospitals and government or other public hospitals. In addition to IRB reviews for medicines and medical devices, reviews for regenerative therapy (*) are available, and you can request EP-Link to provide such support as a single task or in combination with other services.
Experience of Review (October 2022-September 2023): 84 meetings (406 protocols, 679 new trials, 21,525 ongoing trials)
* Certified Committee for Regenerative Medicine since 2017.