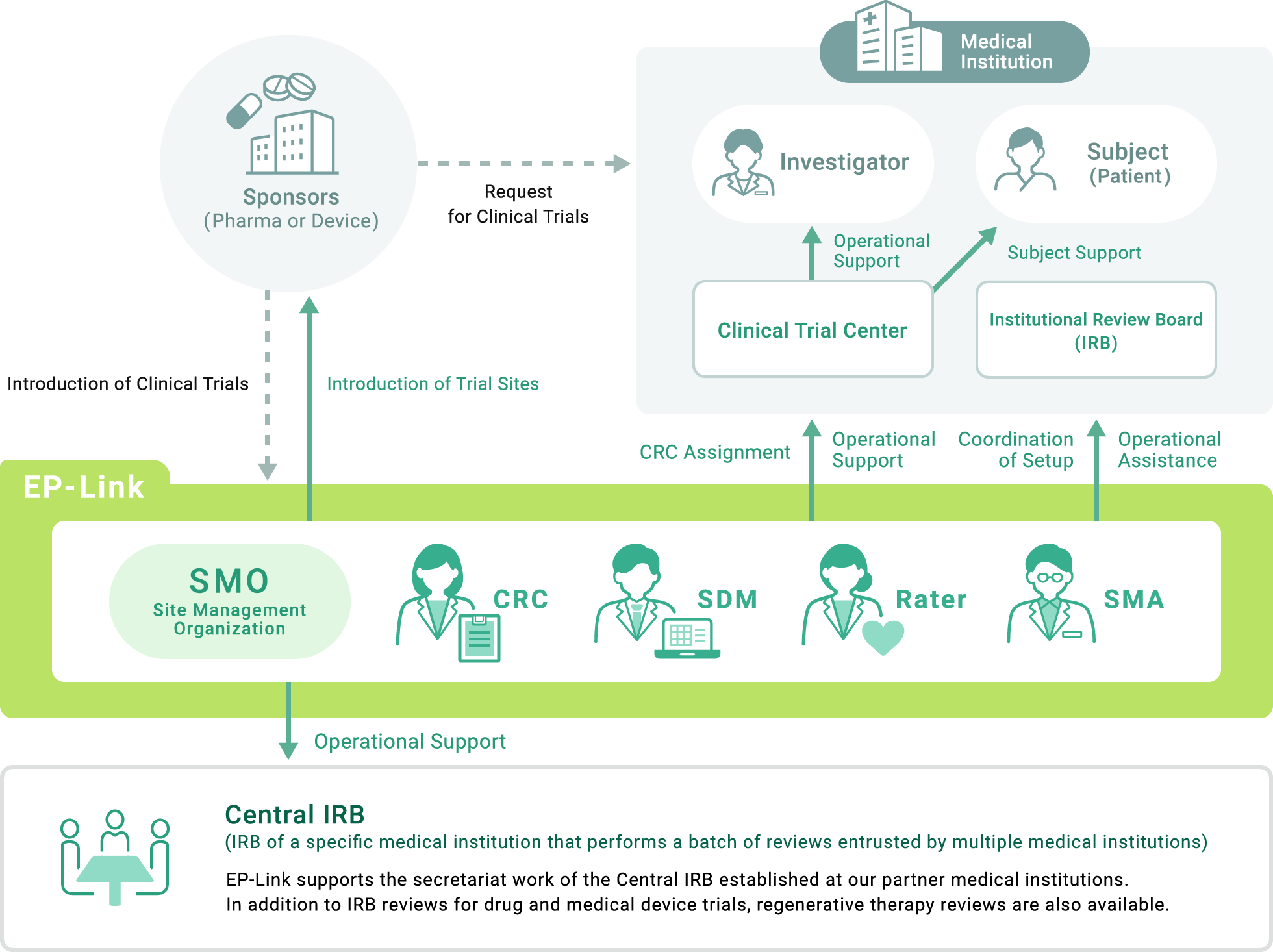
Since the establishment of the Site Management Organization (SMO) business in 1999, EP-Link has acted as a bridge between the pharmaceutical companies that sponsor clinical trials and the medical institutions that conduct the trials, supporting the conduct of clinical trials at medical institutions. EP-Link is committed to responding flexibly to changes in the clinical trial environment, providing optimal solutions to customer needs, and further improving the clinical trial environment.
EP-Link contracts with medical institutions (clinical trial sites) to support clinical trial operations at medical institutions so that they can properly conduct clinical trials in accordance with GCP.
EP-Link supports the work of doctors, nurses, and secretariats involved in clinical trials, reducing the burden and improving the quality and speed.
More than6,000
Click here for Clinical Trial Experience
Approximately7,000
Click here for
Comprehensive Capacities
Management and Promotion
of Enrollment
Click here for Progress
Management System
Quality Cross-organizational
Management System
Click here for Quality
Management System

EP-Link has a large number of CRCs who can play an active role in therapeutic areas that require a high level of expertise, such as oncology, and support high-quality clinical trial work.

SDM leverages its experience as a Clinical Research Associate (CRA) to support data-related operations and help improve data quality in clinical trials.

With a high level of expertise and advanced skills as a clinical trial secretariat, SMAs support the arrangement of clinical trial implementation systems to meet various needs.

Rater Service EP-Link has a large number of assessment psychologists with clinical trial expertise who administer the rating scales required for CNS clinical trials.