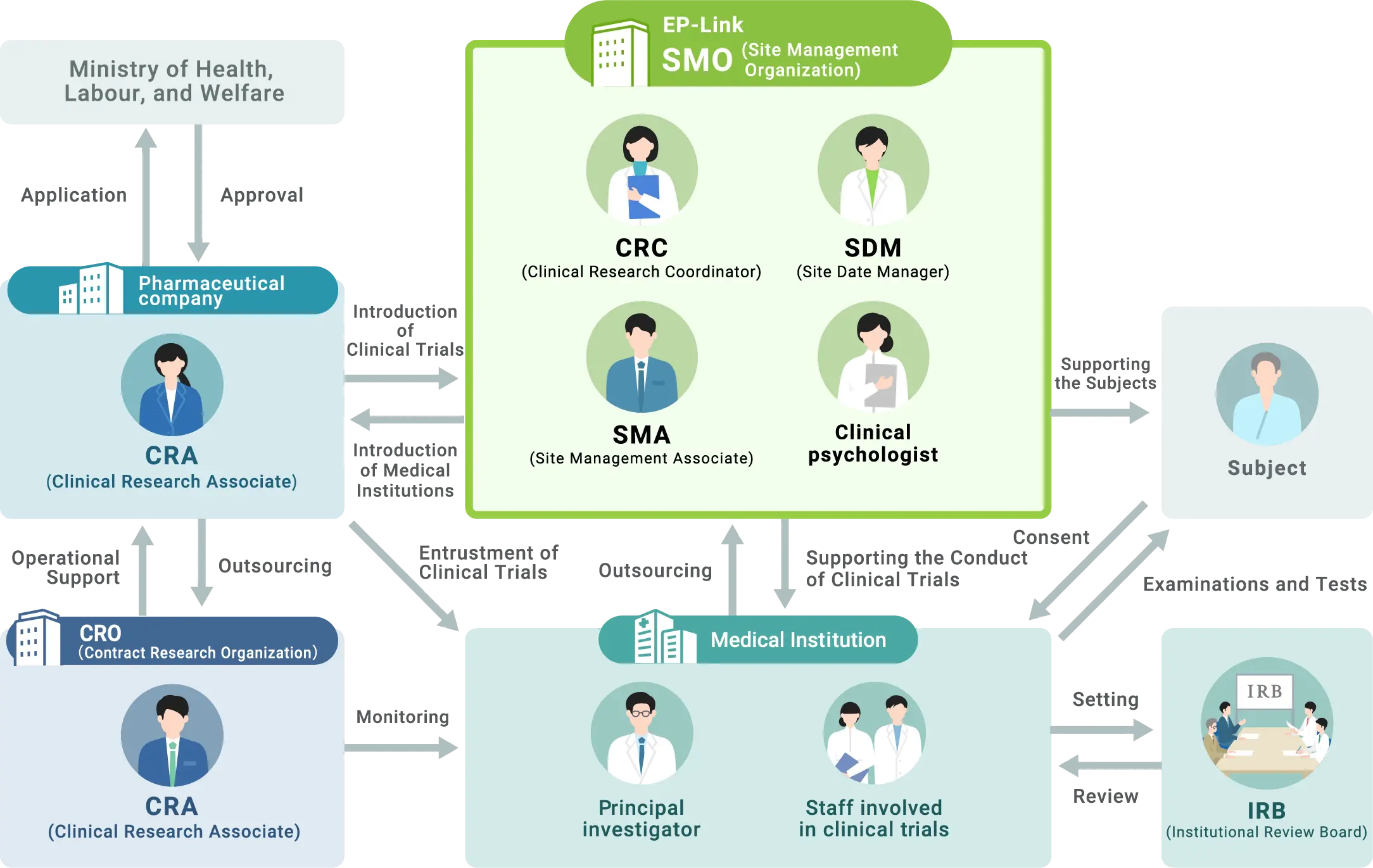
Cooperation among various specialized organizations and professionals is essential to ensure that patients can safely and securely participate in clinical trials and to collect accurate data. Therefore, in addition to the sponsor (pharmaceutical company) and the medical institution conducting the clinical trial, there are also CROs (Contract Research Organizations) that are contracted by the sponsor to perform all or part of the tasks related to the request and management of the clinical trial, SMOs (Site Management Organizations) that are contracted by the medical institution to perform part of the tasks related to the implementation of the clinical trial, and other cooperative organizations beyond organizational boundaries. The clinical trial is conducted under a cooperative framework that transcends organizational boundaries. In addition, an Institutional Review Board (IRB), which is independent of these parties involved in the clinical trial, reviews the safety, ethics, and scientific validity of the trial.
*Click a role to see its description

1,405CRCs

141SMAs

50SDMs

300Psychologists